Formic acid
formic acid (HCO2H), also called methanoic acid, the simplest of the carboxylic acids, used in processing textiles and leather.
Description
Formic or methanoic acid is a colorless organic acid with the formulaHCOOH. At ambient temperatures, it has a strong, penetrating odor, like acetic acid. Used in chemical synthesis as an intermediate, this simple carboxylic acid is miscible in water and most organic solvents and is somewhat hydrocarbon-soluble.
occurs naturally in the venom of some ants and bees. Formate, the conjugate base of formic acid, also occurs naturally in bodily fluids following methanol poisoning.
is a source of hydride ion in synthetic organic chemistry, as in the Eschweiler-Clarke and Leuckart-Wallach reactions. It is also a useful component of the mobile phase in reversed-phase high-performance liquid chromatography (RP-HPLC) for peptides, proteins, and intact viruses.
Although is easily metabolized and eliminated, it can have toxic effects. Methanol metabolism produces formic acid and formaldehyde, which are responsible for the optic nerve damage that results from methanol poisoning. Chronic exposure in humans may cause kidney damage and skin allergies in some people.
In diluted forms, formic acid and formate esters are used as artificial flavorings and perfume additives. In higher concentrations, is flammable, and skin and eye contact with concentrated liquid or vapors is dangerous.
In agriculture, is used as a preservative and antibacterial agent and by beekeepers to kill mites. It is useful in leather tanning, textile dyeing and finishing, and in rubber production. replaces mineral acids in limescale removers and other cleaning products.
Formula
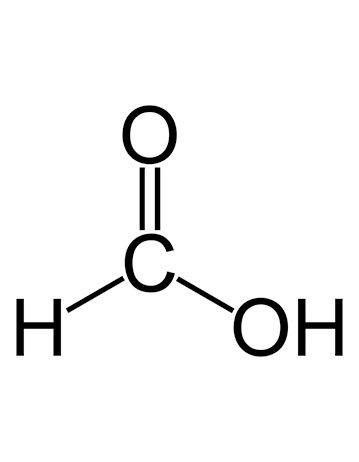
The formula contains one carbon atom, two oxygen atoms, and two hydrogen atoms. Due to the presence of the carboxyl group, its molecular formula becomes HCOOH.
Due to the hydrogen bonding between its two molecules can occur in the form of a dimer.
Structure – HCOOH
As is the first carboxylic acid of the carboxyl family, the structure is simple. Due to the presence of a single carbon atom, the IUPAC name of is methanoic acid.
The structure includes a carbon atom connected with hydrogen by a single bond, oxygen with a double bond, and another single bond with oxygen that is bonded with a hydrogen atom forming a hydroxyl group. You can see the same in the image below, representing the formic acid structure HCOOH.
Properties
The following are some important physical and chemical properties:
Physical Properties of HCOOH
- It is the first member of the identical series of carboxylic acids. Hence, the IUPAC name of is Methanoic acid.
- It is like a thick transparent liquid. Therefore, it is impossible to identify it by its looks.
- The melting point is not very high. It is merely 8.4°C.
- The boiling point is a little over that of water. Its value is 100.8°C.
- Methanoic acid has a low density, a value of 1.22g/cm³, which makes it a less dense liquid.
- The value of formic acid pKa is 3.75. Therefore, it is soluble and miscible with water.
- As it is the first member of the parallel carboxylic acid series, the molecular weight of the formic acid is not very high either. Methanoic acid has 46.03 g/mol of molecular weight.
- Methanoic acid has a characteristic pungent smell and irritating odour.
Chemical Properties of Formic Acid:
- As the name suggests, formic acid is acidic and, therefore, can turn blue litmus paper or solution into red litmus colour.
- Due to the lesser +I effect, formic acid is a hydrogen bond donor molecule.
- Carbon is known to form covalent bonds. As formic acid contains a carbon atom as the central atom, the bonds formed by it are all covalent.
- It can reduce mercuric chloride (HgCl2) into mercurous chloride (Hg2Cl2), resulting in a white residue. The equation for the reaction is given below.
HCOOH + 2HgCl2 → Hg2Cl2 + 2HCl + CO2
- It reacts with phosphoric pentachloride, forming phosphoryl chloride, formyl chloride, and hydrogen chloride. The chemical reaction that happens is given below:
HCOOH + PCl5 → HCOCl + POCl3 + HCl
Methods of Manufacturing Formic Acid
There are two main methods for the preparation of formic acid.
1. Preparation of Methanoic acid in the laboratory
- The dominant laboratory composition of formic acid is heating glycerol and oxalic acid together at 100-110°C.
- The reaction between glycerol and oxalic acid will yield glycerol monoxalate.
- In the next step, this glycerol mono oxalate decomposes to produce carbon dioxide and glycerol monoformate.
- After the expansion of carbon dioxide, some more oxalic acid is added to produce HCOOH.
- The distillate product contains formic acid and water。
The aqueous methanoic acid solution cannot rationalise giving anhydrous acid due to the boiling point of the acid (100.5 °C). Therefore, an aqueous solution can be neutralised with lead carbonate.
Concentrate the solution until lead formate is crystallising. The residue is dried and heated at 100 °C in the presence of hydrogen sulphide. The chemical reaction will be
(HCO2)2Pb + H2S → 2 HCO2H + PbS
The anhydrous acid manufactured by this method contains a small amount of hydrogen sulphide. It is removed by adding dry lead tetraformate and redistilling.
2. Industrial preparation of methanoic acid
The mass production of formic acid occurs at the industrial level by heating sodium hydroxide and carbon monoxide under 6 to 10 atmospheric pressure and 210°C temperature.
NaOH + CO → HCOONa
This reaction takes place at 473K temperature and 6−10atm pressure.
2HCOONa + H2SO4 → 2HCOOH + Na2SO4
Sodium salt prepared in the first step is converted into methanoic acid by distillation.
An aqueous acid solution is obtained by purifying the sodium salt in dilute sulfuric acid. Anhydrous methanoic acid is procured from the molten solution by adding butyl formate pursued by distillation.
Advantages
is the simplest carboxylic acid. Systematically, it is named methanoic acid and has the chemical formula HCOOH. The advantages of formic acid are:
- It is an essential intermediate formed during chemical synthesis.
- It occurs naturally, most notably in some ants.
- Formic acid and its salts are used essentially in the food industry, leather tanning, grass hay, and anti-icing.
- Other industries where formic acid is used are textile, dyeing, finishing, food additives, natural rubber, and drilling.
- Due to its natural antibacterial properties,has been highly used as an antibacterial preservative and pesticide.
- It is frequently added to animal feed and fodder as a food additive. When it is used in fodder, it serves a dual function.
Harmful Effects of Formic Acid
- Strong formic acid solutions are corrosive. Therefore, it can cause burns to any body part when it comes into contact.
- Ingestion of formic acid can result in burns to the mouth, throat, and stomach.
- Difficulty swallowing food, drooling, and vomiting are some other outcomes of consuming formic acid. It may be possible that there is blood in the vomit.
- Eye contact causes pain, watering eyes, twitching eyelids, sensitivity to light, inflammation, and burns are some other harmful effects of frequent contact with formic acid.
- It can irritate the airways and give rise to chest tightness, breathlessness, and wheezing.
Uses of Formic Acid
- Formic acid and citric acid or HCl are used as a mixture. Together, both are strong enough to separate iron oxide deposits.
- Methanoic acid is also used in the manufacturing of important industrial chemicals.
- It is employed as a reducing agent. It can lessen both sodium and potassium dichromate.
- It is a highly functional material in the tanning and dyeing industries.
- Due to its antibacterial properties, is highly used in agriculture.
- It acts as a pesticide and has proven to prevent crops from being attacked by different pests.
Acidity Comparison of Acetic Acid and Formic Acid
An acid’s acidity depends on the carboxylate anion’s stability. The more stable the negative charge, the more acidic the acid (equilibrium will lie more on the dissociated side of the equation). Methyl groups (like in acetic acid) are appraised to be electron donating (by hyperconjugation effect) contrasted to a hydrogen substituent .
In the carboxylate anion, stability is gained by the delocalisation of the negative charge if the methyl group is donating electron density, making the carboxylate less stable than the effect of the hydrogen (none, by definition) in formic acid.
Conclusion
Methanoic acid or formic acid is a corrosive liquid, colourless, pungent smell, and is the simplest carboxylic acid. The chemical formula is H2CO2 or HCOOH. It is a miscible acid that can mix in all proportions with ethanol and water. It is because can form hydrogen bonding with ethanol or water molecules.
In the gaseous form, does not obey the ideal gas law. The reason is simply the establishment of hydrogen bonding. As compared to other monocarboxylic acids like acetic acid, is a stronger acid.
Frequently Asked Questions
1. Is HCOOH safe for human use?
Not in high concentrations. Firstly, might be the metabolic product responsible for methanol toxicity. And secondly, is more acidic as it is a stronger acid than acetic acid. Not only does it cause painful burns, but even blisters on the skin.
2. Why is poisonous?
Metabolism of methanol, esters, methyl ethers and amides gives rise to formic acid. This acid is an interdict of the mitochondrial cytochrome oxidase affecting histotoxic hypoxia. is a weaker interdict than hydrosulphide and cyanide anions. The body load of formate in methanol poisoning is sufficiently high to cause acidosis and more clinical symptoms.
3. Is formic acid a diprotic acid?
No, is not a diprotic acid. Instead, it is a monoprotic acid. Although each formic acid molecule contains two hydrogen atoms, only the hydrogen atom (shown in red below) in the -COOH group is ionisable to hydrogen ions. Hence, is a monoprotic acid.

Reviews
There are no reviews yet.